POLYELITE®
Primary Containers
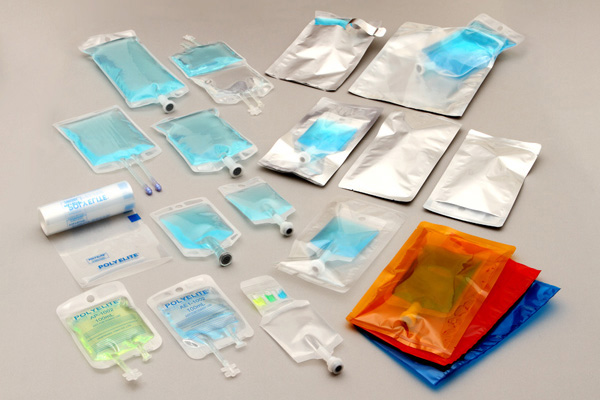
High-quality and Functional ready-to use bags for IV drugs
POLYELITE®
POLYELITE® is the brand of primary packaging for pharmaceutical applications with excellent propeties and safeness.
Compliance of ISO 9001 and 15378
Medix Showa Co., Ltd. has received ISO 15378:2017 certification, which scope is “Design and development, manufacture and distribution of Infusion bag” and ISO 9001:2015 certification which scope is “Design and development, production and distribution of plastic containers”.
POLYELITE® is registered in DMF.

production facility : Medix Showa Co., Ltd. (Nagano, Japan)
POLYELITE® is manufactured in the ISO class 7 clean room.
Products
Polyethylene Bag for Steam Sterilization

POLYELITE® TH82
- Made of polyethylene resins only
- Applicable to a wide range of temperatures from -80℃ to +123℃
- Excellent mechanical properties at very low temperature
- Extremely low extractables and leachables
- JP 7.02-2.1 Polyethylene or Polypropylene Containers for Aqueous Injection
- USP<661.2> Plastic Packaging System for Pharmaceutical Use
- USP<87> Biological Reactivity Tests, in vitro
- USP<88> Biological Reactivity Test, in vivo, Plastic Class VI
- USP<85> Bacterial Endotoxins Test
- USP<788> Particulate matter in injections
- EP 3.2.2.1. Plastic Containers for Aqueous Solutions for Infusion
Sorption-free Bag for Steam Sterilization

POLYELITE® PHC
- Cyclo-olefin polymer layer as a contact layer
- Extremely low sorption of lipid-soluble drug substances
- Excellent transparency after steam sterilization
- Extremely low extractables and leachables
- JP 7.02-2.1 Polyethylene or Polypropylene Containers for Aqueous Injection
- USP<661.2> Plastic Packaging System for Pharmaceutical Use
- USP<87> Biological Reactivity Tests, in vitro
- USP<88> Biological Reactivity Test, in vivo, Plastic Class VI
- USP<85> Bacterial Endotoxins Test
- USP<788> Particulate matter in injections
- EP 3.2.2.1. Plastic Containers for Aqueous Solutions for Infusion
Polyethylene Bag for Bio-pharmaceuticals

POLYELITE® EL
- Additive-free polyethylene film and bag
- Excellent mechanical properties at very low temperature
- Applicable to storage at liquid nitrogen temperature
- Extremely low extractables and leachables
- JP Polyethylene or polypropylene containers for aqueous injections
- USP <661> Physicochemical tests
- USP <87> Biological reactivity tests, in vitro
- USP <88> Biological reactivity tests, in vivo; Plastic Class VI



